How to weigh the advantage of features
It is quite a complicated task to arrive at conclusion when you come across different models with several features and specifications, each company stirring at their own U.S.P. sometimes they are totally irrelevant & at times they are not truly significant to your work – even if taking futuristic requirements in mind. If they are given to you without any extra cost it is not a problem but if some cost is attached to it, then definitely a due though required.
Actually, there is a very simple technique which can be used effectively. Any feature for that matter, just ask one question to yourself “So What? What is in it of me”? If the feature is having certain advantage look for the real benefit you are getting out of it.

When a designer develops a certain product he goes by various ways. Although human brains work differently, the basic requirements are same – they have to be there. But the intention of these entirely depend upon the imagination and understanding of the users requirement in depth. And then come the sales gimmicks!
Further it also depends upon what technology he is using. And that’s why & where users get confused. Users many times are unable to identify the inside story. They may get carried away by many unwanted specifications. With PC or microcontroller it is easy to give wide range of measurement irrespective of basic limitations. For example pH range will be shown as 0-20 pH or temperature will be shown as ambient to 999°C. Such specifications are generally kept to eliminate certain unfortunate manufactures. Now let us see some of the features and also see their advantages and benefits.
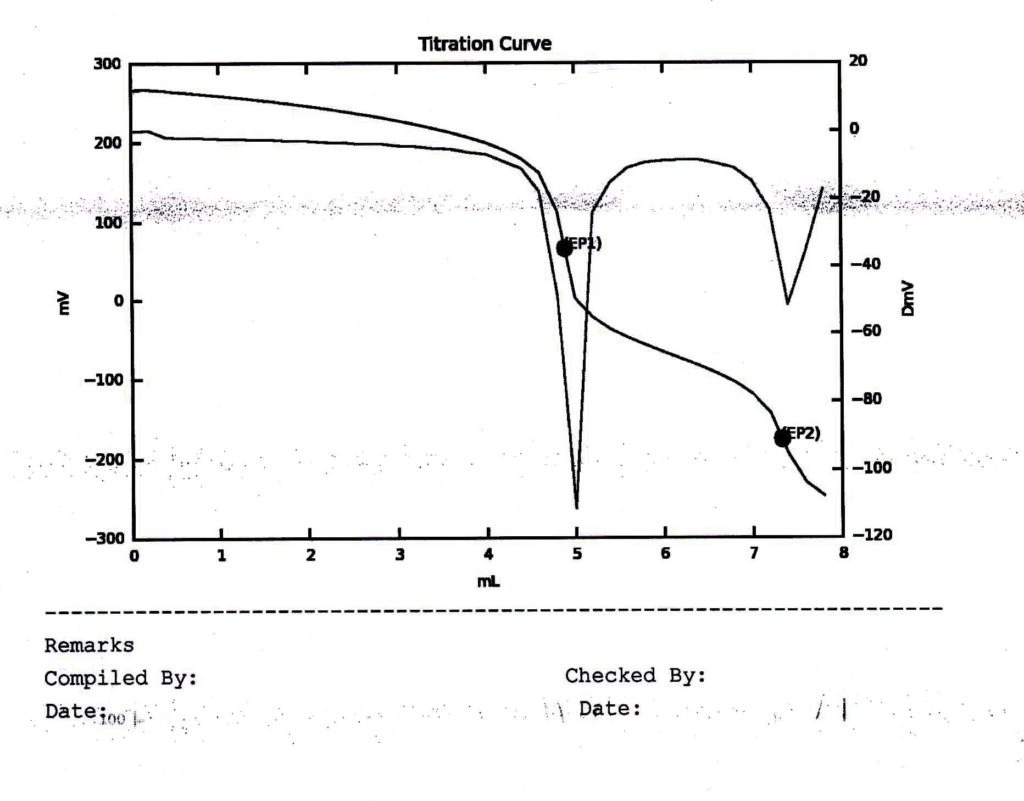
On Line Curve : By this feature the operator is able to see how the titration is progressing. This will also give derivate curve simultaneously, indicates where are the significant peaks.
Just ask yourself what benefit you are getting out of this feature. Well, YES it is very useful to have advance intimation before you actually get the result. If this benefit appeals to you, you can certainly increase your budget.
Automatic Burette Recognition :
By this feature you can ensure which titrant you are using. This has a definite advantage as you can know the contents like name & normality of the titrant well before you start the titration. The benefit you get is avoiding wastage of time & chemicals. But still you can ask yourself – does this feature is beneficial to you. If yes surely you can go for the model which will give this facility to you.
However, if you are having just one or two types of titrations, there is not much substance for this feature. At the same time, where you are having wide range of titrants, it is of advantage to have more numbers of burette available with you to use the above feature effectively. Sometimes people go for the model with such facility but they tend to be compromising on number of such extra interchangeable burette assemblies. This is not a wise choice. This way the very purpose of having automatic burette recognition facility is lost.
“Stand alone” model or “PC through operation”:
This is another feature where you stand at the dilemma. Actually having a standalone model where data can be transferred to PC is also a good feature as you do not have to make provision in your budget for PC, added requirement of table space to keep PC. However you have to ensure that it has a facility to print using the printer available in the market. Also make sure that it has a facility to take the data on pen drive so that you can take to nearby PC where you can transfer the data.
You have advantage & limitations on both the types but you have to decide for your best It is also advisable to check if the stand alone model can be further upgraded in future for PC through operation.
Now a days some manufacturers give facility of “on line “support. This is very useful feature because you can get instant support. The supplier’s service engineer cannot be present at each and every location. You have to give some consideration to this reality. Although there may not be a fault with the instrument, but there may be a problem with the operator. He might change the job and someone else might have joined, he may not be trained or anything for that matter. The “On Line “support will considerably keep the down time under control unless of course there is some hardware / mechanical problem, but 90 % of the problems are associated with things which are “Outside” of the instrument. If your lab is in remote location, you have to give considerable weight age for such facility.
Moisture Estimation using Karl Fischer Titrator:
Quite often this facility is given as an option. Whether to go for this add on or procure a separate titrator for moisture estimation is creating dilemma. Simple thumb rule is to check the proportion of work and how frequently you are required to carry out it. And decide. However if the titrator is already to be used for variety of titrants adding KF facility also makes the things bit complicated. The titration using the Karl Fischer titrator is somewhat different than other potentiometric titrations, in the sense that it requires neutralization. Hence every time you switch over, there is wastage of time & chemicals like Methanol & KF reagent. Although initial cost of two separate Karl Fischer titrators may make you inclined towards less costly option it will have above draw backs. In all practical purpose two separate instruments is a better choice.
pH stat titrations :
Unless otherwise specifically do not go for this as an attachment. Keeping the titrator decided to this work is a better option. pH Stat requires long duration work and if the titrator is all the time engaged for pH Stat, other samples will wait. Further pH Stat is temperature controlled titration. Ensure that a heating / cooling system is available for maintaining desired temperature with accuracy. It should be compact so that it will not look clumsy, at the same time achieving the desired temperature quickly enough. Large size water baths will not work as they take long time. A solid state cooling / heating bath will be quite useful as it occupies less bench space.
Method Development:
Method development is bit tedious and yet important aspects of titration. It is dependent on various factors and the instrument adopted for the same should satisfy certain requirements such as dose steps, variable endpoint sensitivity, ml Vs mv report, variable endpoint criteria, dead stop criteria, start criteria for more than one step, Endpoint anticipation for unknown sample and last but not the least a user defined formula apart from the standard formulas.
The repost generated from the instrument also plays a vital role in method development and validation. The report generated from the equipment should be such that even a lay man can understand the details and proceed accordingly.